
A science-backed guide to the ancient practice of building a campfire.
Before the invention of clothing, agriculture, and even the wheel, our ancestors were playing with fire.
How do we know this? In 2012, archaeologists announced they had uncovered traces of ash, burnt twigs, and animal bone — evidence of a controlled fire — while excavating a cave in South Africa. Those tiny fragments were more than a million years old and likely the handiwork of Homo erectus, a species that predates Homo sapiens (i.e., you and me).
Not only did this evidence suggest our ancestors invented campfire before they invented anything else (perhaps with the exception of stone tools), it also revealed that making fire was one of the very first activities to get us working together.
Yet this incredibly ancient practice of campfire making still remains mystifying to many of us humans. A few months ago, a group of friends and I shared a laugh around a campfire realizing none of us — college-educated adults — could explain what, exactly, fire was.
Considering fire’s importance in human history — and that it’s still how most of the developing world keeps warm and cooks food — we really should understand it.
Here’s a science-backed history and guide to the ancient practice of building a campfire, from its importance for human evolution to the chemistry of how it burns to this age-old fuel’s impact on our health and our environment.
Campfire was monumentally important to our evolution
 Mathias Erhart / Flickr
Mathias Erhart / Flickr“Campsites are in essence nests made by human beings,” the biologist E.O. Wilson writes in The Social Conquest of Earth. And nests are the first step to becoming a species whose members look out for one another. The earliest humans “raised young in the nest, foraged away from it for food, and brought back the bounty to share with others,” Wilson writes. The campfire is the origin of community.
It’s also possible that fire aided our cognitive development. In the book Catching Fire, biological anthropologist Richard Wrangham argues that campfires — and the subsequent invention of cooking meat and eating it — were the catalyst that allowed our ancestors to develop big brains. “The extra energy [in the cooked meat] gave the first cooks biological advantages,” Wrangham writes. “They survived and reproduced better than before. Their genes spread. … There were changes in anatomy, physiology, ecology, life history, psychology, and society.”
In these early days, it’s likely our ancestors didn’t actually know how to start fires. They only knew how to maintain them — after a lightning strike or spontaneous conflagration of brush got one started. Anthropologist Christopher Dana Lynn writes in the journal Evolutionary Psychology that inability to start fires put even more pressure on early humans to cultivate ingenuity and tolerance for one another:
The inability to start fires would have required groups to coordinate activities to access and maintain them. This continual cooperation would have put pressure on cognitive capacities for social tolerance, conceiving of others as collaborators in future cooperation …
Dana Lynn also hypothesizes that we may be instinctually comforted by fire; he found some preliminary evidence that suggests blood pressure lowers when we’re around it.
Fire would also lead to inventions like the steel mill and the steam engine, which would allow humans to literally reshape the world to their likings.
“Fire is the one [invention] that made us,” Adrian Bejan, a thermodynamics professor at Duke and the author of The Physics of Life, a new book on technological evolution, tells me. “Without fire you and I would be nothing today.”
So what, exactly, is fire?
 rahul rekapalli / Flickr
rahul rekapalli / FlickrWhen you look at flames, you are seeing the results of a complex chemical reaction called pyrolysis. You’re seeing wood turned into gas, gas ignited by heat, and light from the excitement of electrons.
Here’s another way to think about it: The entire process of a fire is about tearing a log into as many pieces as possible. The tearing releases chemical bonds, expending energy as heat and light.
But anyone who has tried to ignite a whole log with just a single match knows that it takes a lot to get a fire going. You can’t do it with a single match or spark from a piece of steel on flint.
You have to take a tiny bit of energy and transform it into a self-sustaining reaction. Each component of the wood has to absorb enough heat to begin the pyrolysis process.
Here’s how it goes: As plant fibers heat up, the plant’s tissues — mostly made out of a molecule called cellulose — start degrade and break down. As the tissue gets hotter and hotter, water is driven out of the cells, and they then break apart, forming volatile, combustible gases, “just like a burner from your stove,” John Bailey, a professor who teaches fire science Oregon State, tells me.
Igniting those gases releases some energy, which then can be used to break down more cellulose and generate more combustible gases and heat.
/cdn.vox-cdn.com/uploads/chorus_asset/file/6806735/Screen%20Shot%202016-07-18%20at%201.59.36%20PM.png) Bioenergy
BioenergyAll of this needs to be done in the presence of oxygen, as fire is an oxidation reaction. (The oxygen bonds with the carbon in the wood to form carbon dioxide, and releases heat and water along the way.)
The ignition of the gas continues the process of breaking down that log further and further. Inside that gas are actually hundreds of carbon-based compounds. Some of these form soot and then are broken down further in the flame. If a fire burns perfectly, the log will break down all the big molecules into carbon dioxide and water vapor.
But why does this process create light?
It comes from the electrons releasing extra energy — going from an exited state to a less excited state. (You know how metal glows when it’s heated red-hot? The same thing is happening in the flame, but instead of metal, it’s the tiny particles of soot absorbing the energy.)
Here, via the YouTube channel Distort, is a very cool look at how pyrolysis occurs in slow motion.
How do I build a campfire?
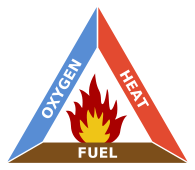
Building a campfire only takes three ingredients.
Fuel: Twigs, paper, branches, logs, etc.
Oxygen: Free and always available!
Heat: A match should do the trick.
These three are called the “fire triangle,” and if they are kept in balance, a campfire will burn evenly and thoroughly. But if they are out of whack, the fire will sputter and fail.
 Wikimedia Commons
Wikimedia CommonsHere are some general rules to get a good campfire going.
1) The first rule of building a campfire is to start small.
You may be tempted to start with big branches if you have a big fire in mind. But remember, a match or a lighter contains only a tiny speck of energy — just enough to bring small branches, pine needles, or other kindling to the critical temperature at which pyrolysis can occur.
As a campfire builder, it’s your job to coax that tiny bit of energy into growing, slowly and thoroughly. So start with some very dry kindling, some pine needles, and crumbled-up bits of your least favorite daily newspaper.
Also remember: Large pieces of wood have to absorb a lot of energy before they ignite. If there’s too little heat in the fire, they won’t catch. Too much wood will smother the flame from oxygen as well.
2) Use the driest branches you can find.
Be sure to find the driest pieces of wood for your campfire, as it will take extra energy to get wet pieces of wood to start burning. “If the moisture content is too high, an appreciable amount of energy is necessary to vaporize the water, reducing the heating value of the wood as well as decreasing combustion efficiency, which in turn increases smoke formation,” the journal Chemosphere explains. Wet wood will also produce more smoke, which is bad for your health and environment (more on that below).
When the fire is hot enough — when there’s an inch or two of hot, glowing coals on the bottom — it won’t matter if the fuel is a bit wet. “If you build up a nice bed of coals, you’ve got the fire getting progressively bigger, you’ve got this big generating heat source now, then you can lay that big piece on there,” Bailey says.
3) Start with low–density wood.
The best woods to get a campfire going are something light, like pine or cedar. These will ignite the fastest. (Pine also has a lot of resin, which makes for nice crackles when in a fire.) Then move on to denser woods, like oak. These take a lot more energy to start burning and will burn longer.
When it gets going, a fire is like a living thing. It needs to be fed, sustained, and looked after, or it will die.
4) The best shape for a fire is one that’s as tall as it is wide.
You can find plenty of diagrams for building campfires: teepee designs, log cabin designs, elaborate plans for digging underground air-intake vents. But really, all these designs come back to one basic rule: Build a fire that’s as tall as it is wide.
This rule comes from Bejan, who thought of it while watching a mound of charcoal ignite in his backyard grill. He realized when a fire is built into a pyramid shape, it will burn the hottest for the longest amount of time. It’s the perfect trade-off.
/cdn.vox-cdn.com/uploads/chorus_asset/file/6770205/1433952906-1291094392910535269%20(1).jpg) Duke
Duke“If the shape of the fire is extremely flat, shallow, then the fire does not draw [oxygen],” Bejan explains. “A flat fire means a puny, puny fire. The extreme is to have a skinny, stick-like pile. The skinny stick is burning all right, but it is so skinny, it’s surrounded by cold air; therefore, it is a cold fire. That, too, is a bad design.”
In the middle is “a very special shape where the cone is not too shallow, not too tall, and the answer is the base is as wide as it is tall,” he says.
Bejan published this finding in the journal Scientific Reports. To him, the universality of the fire shape is evidence that humans have an innate sense of physics. “There are many stupid ways of making fire, but there’s one special way of making it, which needs no explaining, because everybody knows it,” he says. That’s universal across human cultures, stretching far back to the beginning of time.
But wait, isn’t it bad to breathe in campfire smoke?
Yes!
If a fire burned perfectly, the log would be completely torn down into carbon dioxide and water vapor.
But most fires do not burn perfectly. And as a result, wood smoke contains a lot of pollutants: chemicals like benzene and formaldehyde, as well as fine particles that can irritate lungs and eyes. As Brad Plumer has explained, indoor air pollution from wood smoke is the deadliest environmental hazard on the planet.
But the hotter a fire burns, the more these toxic chemicals can get broken down into simpler, safer ones. Burning dry wood also helps keep these pollutants to a minimum. But no matter what, “you’re going to put out a lot of pollution when you burn a campfire,” Lisa Herschberger, an environmental research scientist with Minnesota’s pollution control agency, says. “I would stay upwind of it.”
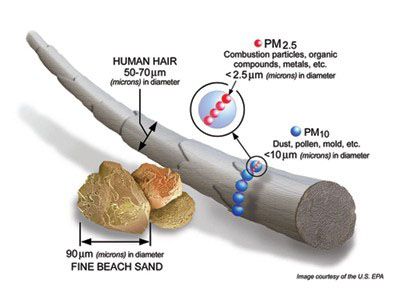
Fine particles from the smoke and soot can be smaller than 2.5 micrometers — tiny enough to lodge themselves into the crannies of the lungs.
“The biggest health threat from smoke comes from fine particles,” the Environmental Protection Agency warns. “These microscopic particles can get into your eyes and respiratory system, where they can cause health problems such as burning eyes, runny nose, and illnesses such as bronchitis. Fine particles also can aggravate chronic heart and lung diseases — and even are linked to premature deaths in people with these conditions.”
What about the environment? Is wood smoke a pollutant?
The particles from wood smoke also can contribute to smog and haze. In Minnesota, for instance, where recreational outdoor fires are popular, Herschberger says recreational wood smoke accounts for around 5 percent of all the fine particles released to the air. “I call this a sizable contribution to air emissions,” she says.
In terms of carbon dioxide emissions, wood smoke can be carbon neutral if the wood you burn is replaced by new growth. “But it’s not a slam dunk,” Herschberger says. “It will be really important [for emissions] to learn how that wood was grown, how it was transported. It takes knowing the whole life cycle of the wood to know if you’re ahead or behind [on carbon emissions].” Burning dead wood also releases what’s known as black carbon, or soot, which has a greater warming impact than carbon dioxide alone, she says.
Another danger is when fires grow out of control.
On the small scale, fire is predictable. “If you build exactly the same pile of kindling and had the same temperature, you would get literally the same fire again. It’s just physics and chemistry,” Bailey says. But when it sparks into a forest fire, it’s an entirely different beast.
“The fuels get very complicated, and the temperature, wind speed, relative humidity varies,” he says. “Then you just have random stuff: a gust of wind, a pine cone rolling down a hill, a little stream flowing through an area. It makes fire on a mountainside absolutely magical, unpredictable.”
(Like dark magic? “The more you’re fascinated by fire, the more it moves from the dark to the light,” he says.)
With ongoing drought, climate change, and, ironically, a history of fire suppression, forest fires in the Western US have been growing bigger and more destructive over time.
A lot of these fires are started by lightning. But as Smokey the Bear has cried for decades, you can help prevent forest fires.
Bailey offers a few commonsense suggestions:
- Don’t leave a fire unattended. Put out fires with water, or smother them completely before heading out of a campsite.
- Don’t park a car on dry grass. Car underbodies can get hot enough to ignite fires.
- Be aware of fire hazard warnings before lighting a fire in a forest.
A final mystery: Why do marshmallows catch fire?
 monnibo / Flickr
monnibo / FlickrA marshmallow is made up of gelatin and sugar. Sugar burns at around 350 degrees Fahrenheit. A campfire can top more than 1,000 degrees. That’s why it takes Jedi concentration to heat a marshmallow over a flame (but not too close!) so it oozes instead of cinders.
Read More
https://cdn.vox-cdn.com/community_logos/52517/voxv.png


