The clinics claim they can treat serious diseases with stem cells from fat. The FDA doesn’t buy it.
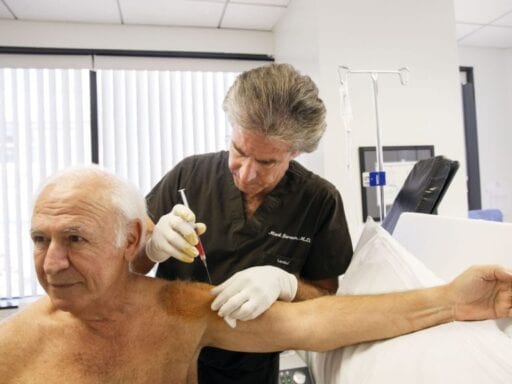
Inside Mark Berman’s clinic in Rancho Mirage, California, is a sign he’s obliged by law to post. It reads “Not FDA Approved.”
Patients who come here to the California Stem Cell Treatment Center can get treatments for ailments ranging from sports injuries to muscular dystrophy. For upward of $5,000, Berman, a plastic surgeon by training, will remove a small portion of their fat, process it, and inject it back into them.
This is called “fat-derived stem cell therapy”; the premise is that the stem cells in your fat can jump-start the healing process. “The stem cells could be good for repairing everything from Alzheimer’s to paralysis to neurodegenerative conditions,” says Berman. “These cells are miraculous for helping heal. We don’t have a choice. We have to use them.”
The problem is there’s not much evidence to back up the claims Berman is making. And it’s not just him — there are more than 100 clinicians in the Cell Surgical Network, a group he co-founded in 2010 to promote the same kind of adult stem cell regenerative medicine he practices. According to a 2017 report by three Food and Drug Administration scientists in the New England Journal of Medicine looking at the benefits and risks of this kind of stem cell therapy, “This lack of evidence is worrisome.”
Fat-derived stem cells “may have a positive effect,” says Brad Olwin, a professor of molecular cellular and developmental biology at the University of Colorado Boulder with more than 30 years of experience working with stem cells. “They may be beneficial; it’s clearly a possibility. The problem is the research hasn’t been done.”
So little evidence exists, in fact, that the Department of Justice, on behalf of the FDA, is suing Berman’s clinic as well as a clinic in Florida for experimenting on patients with misleading products. The complaint was filed in May 2018 and the investigation is ongoing, according to the DOJ.
Given the popularity and abundance of these clinics nationwide, the FDA is also taking steps to modernize regulation in the field. But despite these efforts to streamline a path to legitimacy for stem cell clinics, unregulated medical procedures persist, at times leading to patient harm.
/cdn.vox-cdn.com/uploads/chorus_asset/file/13676530/GettyImages_818637778.jpg) Patrick T. Fallon for The Washington Post/Getty Images
Patrick T. Fallon for The Washington Post/Getty ImagesClinics recruit and treat patients despite warnings from the FDA and bioethicists
Currently, the only stem cell therapies approved by the FDA use cells from bone marrow or cord blood to treat cancers of the blood and bone marrow.
But doctors in the Cell Surgical Network have moved ahead with using cells for autoimmune, neurologic, and other serious conditions.
And there is a growing number of cases of adverse effects. In 2016, an elderly woman went blind after receiving an injection of stem cells to treat her macular degeneration. She received the treatment at the Stem Cell Center of Georgia — an affiliate of Berman’s Cell Surgical Network.
More reports of ill-fated procedures have since surfaced across the country, the worst resulting in kidney failure and paraplegia. In December, the Centers for Disease Control and Prevention reported 12 cases of people who suffered bacterial infections from contaminated stem cell treatments. An investigation traced the infections back to a single provider, Genetech, prompting the FDA to issue a warning letter to the company. FDA Commissioner Scott Gottlieb then issued a public statement reaffirming the agency’s intent to regulate unapproved treatments.
Bioethicists are sounding the alarm too. In a recent paper in the journal Perspectives in Biology and Medicine, the University of Minnesota’s Leigh Turner examined the marketing claims of 716 stem cell clinics in the United States. Often, he found, they were misleading. “What at first glance might appear to be credible and compliant clinical research often is highly problematic,” he wrote, adding that the individuals most affected are those “who often are already dealing with serious health problems and other challenges.”
Despite two years of increased scrutiny from the FDA, clinics continue to recruit new patients. Berman insists that repurposed fat-derived stem cells should not be subject to the same regulations as other treatments, and that FDA guidelines are arbitrary and nonscientific. “They are a violation of constitutional rights to your own property.”
He noted that after the case of the woman with macular degeneration going blind, his network’s clinicians no longer inject fat-derived stem cells into patients’ eyes. But they continue to offer intravenous (bloodstream) injections. “We have virtually three or four adverse events, of very little significance or consequence,” says Berman, referring to the patients in his network. But according to the FDA, intravenous injections are “associated with higher risk.”
Other scientists I spoke with say they’re also worried that intravenous treatments may harm patients. “You’re taking cells out of one part of your body, and putting them into another. You have absolutely no control after that,” says Olwin. He acknowledges the FDA’s efforts to crack down on clinics but suggests that much more can be done. “They have limited resources to go after people. But I think it’s irresponsible for doctors and these clinics to be promoting these things.”
The disease-treating value of fat-derived stem cells lacks evidence
Some types of stem cells can indeed give rise to virtually any cell in the body — providing a platform for regenerating muscle or even rebuilding organs. Stem cells derived from embryos have this power, called pluripotency, but those obtained from adults do not. In order to acquire pluripotency, adult stem cells must be biologically reprogrammed — a feat that, when invented, led to a Nobel Prize. These induced pluripotent stem cells allow doctors to treat challenging illnesses such as leukemia.
But clinics like Berman’s are not using pluripotent stem cells — they are using the mesenchymal stem cells found in fat, which are much more limited in function. Arnold Caplan, the field’s pioneer who first gave them the “stem cell” label, recently advocated for renaming them to prevent doctors from claiming that they “can cure the blind, make the lame walk, and make old tissue young again.”
BrainStorm, a biotechnology company working with mesenchymal cells, recently gained FDA approval to begin clinical trials to treat patients suffering from multiple sclerosis. But to treat the neurological condition, BrainStorm researchers have developed a method to convert the mesenchymal cells into “biological factories” that release disease-treating proteins. In other words, BrainStorm’s therapy doesn’t involve mesenchymal cells doing the work on their own — what some clinics in the Cell Surgical Network claim mesenchymal cells can do.
Outside of the Cell Surgical Network, other clinics are using patients’ fat-derived cells but making different claims about the treatment.
“I don’t say I’m doing stem cell therapy,” says Dr. Joanne Halbrecht, an orthopedic surgeon and founder of Boulder Regenerative Medicine. Her clinic uses patients’ fat-derived cells to treat orthopedic conditions, injecting them into joints.
Halbrecht avoids the “stem cell” label because current research does not support claims that these fat-derived cells can turn into cartilage. Instead, she uses patients’ fat to cushion their joints. According to Olwin and the FDA, such joint injections are significantly lower-risk than intravenous injections.
Berman also administers direct joint injections. But afterward, he tells me, his clinicians also inject the leftover cells into the patient’s bloodstream. Halbrecht is adamant that this kind of procedure is unproven and unsafe.
“That’s definitive. It’s not a question,” she says. “They are completely wrong because there is zero science behind that.”
The FDA is offering doctors a new path to testing stem cell therapies
For clinics to prove the safety and efficacy of their fat-derived stem cell treatments to the FDA, they must run rigorous clinical trials.
But some clinicians argue that even if they were interested in clinical trials, getting the FDA’s blessing is too daunting. Clinical trials span years and cost millions of dollars. For small, privately owned clinics, this process is unaffordable.
In response, the FDA unveiled a more feasible clinical trial process, better suited to small businesses. Clinics that want to test a specific treatment can now team up on clinical trials and pool their patients, which can save them time and money. Still, the FDA is offering a grace period of up to 36 months for clinics to comply with its guidelines, allowing many to continue operating on patients without doing clinical trials. In the meantime, the FDA is urging patients to “do [their] part to stay safe,” according to a consumer warning issued in May.
Unfortunately, that’s not so easy. Whether or not a clinic is offering an FDA-compliant treatment can be unclear. Some doctors advertise compliance because the device they use to remove and process a patient’s fat is technically FDA-approved. But if they then advertise their treatment as an “FDA-approved” stem cell therapy, they risk misleading patients.
Berman has no plans to pursue clinical trials, even with the new streamlined process. He believes his current model of clinical experimentation is adequate. In the so-called “safety studies,” he treats paying patients with a wide variety of diseases. But according to the recent bioethics report, Turner found that these pay-to-participate studies are poorly designed and unscientific.
In Berman’s view, more patients benefit by obtaining cutting-edge treatments faster. But for every revolutionary treatment developed in a lab, there are nine duds and many unpredictable dangers. And unsanctioned clinics cost patients thousands of dollars and are not covered by insurance. Critics argue that it is unethical to charge patients for experimental procedures, as sanctioned clinical trials rarely cost patients anything.
The economic incentives for unsanctioned stem cell clinics are clear. Starting clinical trials would not only reduce patient revenue but also commit clinics to a costly process known to last for years. Shifting blame to the government and research community, Berman assures me that he and his colleagues are not motivated by self-interest. “We’re the good guys,” he says.
In March, the woman blinded by an unsanctioned stem cell treatment filed a lawsuit against Berman’s Cell Surgical Network. Berman’s site still advertises treatment for macular degeneration with a link to an application.
But tucked away on Berman’s website sits a sort of confession that may surprise the many patients who hear him speak with unwavering assurance. The page reads, “We do not claim that these treatments work for any listed nor unlisted condition, intended or implied.”
Max Levy is a PhD student in chemical and biological engineering at the University of Colorado Boulder and the senior editor of Science Buffs, a graduate student science blog. He writes about health, medicine, and the environment.
Update, January 11: A reader pointed out that the FDA has also approved stem cell treatments from cord blood and so the post has been updated to reflect that.
Author: Max Levy
Read More