It could revolutionize everything from medicine to agriculture. Better read up now.
One of the biggest and most important science stories of the last few years will probably also be one of the biggest science stories of the next few years. So this is as good a time as any to get acquainted with the powerful new gene-editing technology known as CRISPR.
If you haven’t heard of CRISPR yet, the short explanation goes like this: In the past six years, scientists have figured out how to exploit a quirk in the immune systems of bacteria to edit genes in other organisms — plant genes, mouse genes, even human genes. With CRISPR, they can now make these edits quickly and cheaply, in days rather than weeks or months. (The technology is often known as CRISPR/Cas9, but we’ll stick with CRISPR, pronounced “crisper.”)
Let that sink in. We’re talking about a powerful new tool to control what genes get expressed in plants, animals, and even humans. The ability to delete undesirable traits and, potentially, add desirable traits with more precision than ever before.
In 2017 alone, researchers reported in Nature that they’d successfully used CRISPR in human embryos to fix a mutation that causes a terrible heart muscle disorder called hypertrophic cardiomyopathy. (Other researchers have since called some of the conclusions into question.) Another team used it to reduce the severity of genetic deafness in mice, suggesting it could one day be used to treat the same type of hearing loss in people.
Meanwhile, researchers at the Broad Institute of MIT and Harvard launched a coordinated blitz with two big studies that move CRISPR in that safer and more precise direction. A paper published in Science describes an entirely new CRISPR-based gene editing tool that targets RNA, DNA’s sister, allowing for transient changes to genetic material. In Nature, scientists published on a more refined type of CRISPR gene editing that can alter a single bit of DNA without cutting it — increasing the tool’s precision and efficiency.
And these are just a few of the astounding things researchers have recently shown CRISPR can do. We’ve already learned that it can help us create mushrooms that don’t brown easily and edit bone marrow cells in mice to treat sickle-cell anemia. Down the road, CRISPR might help us develop drought-tolerant crops and create powerful new antibiotics. CRISPR might one day even allow us to wipe out entire populations of malaria-spreading mosquitoes or resurrect once-extinct species like the passenger pigeon.
But there are real limits to what CRISPR can do, at least right now. Scientists have recently learned that the approach to gene editing can inadvertently wipe out and rearrange large swathes of DNA, in ways that may imperil human health. That follows recent studies showing CRISPR-edited cells can inadvertently trigger cancer.
As scientists work to overcome these limitations, much of the hype around CRISPR has focused on whether we might engineer humans with specific genetic traits (like heightened intelligence). But in some ways, that’s a sideshow. “Designer babies” are still far off, and there are enormous obstacles to making those sorts of complex genetic modifications. The stuff that’s closer at hand — from new therapies to fighting malaria — is what’s most exciting. So here’s a basic guide to what CRISPR is and what it can do.
What the heck is CRISPR, anyway?
If we want to understand CRISPR, we should go back to 1987, when Japanese scientists studying E. coli first came across some unusual repeating sequences in the bacteria’s DNA. “The biological significance of these sequences,” they wrote, “is unknown.” Over time, other researchers found similar clusters in the DNA of other bacteria (and archaea). They gave these sequences a name: Clustered Regularly Interspaced Short Palindromic Repeats — or CRISPR.
Yet these CRISPR sequences were mostly a mystery until 2007, when food scientists studying the Streptococcus bacteria used to make yogurt showed how these odd clusters actually served a vital function: They’re part of the bacteria’s immune system.
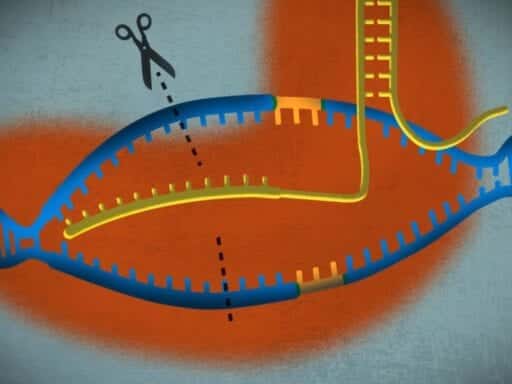
See, bacteria are under constant assault from viruses and produce enzymes to fight off viral infections. Whenever the bacteria’s enzymes manage to kill off an invading virus, other little enzymes will come along, scoop up the remains of the virus’s genetic code, cut it up into little bits, and then store it in those CRISPR spaces.
Now comes the clever part: The bacteria uses the genetic information stored in these CRISPR spaces to fend off future attacks. When a new infection occurs, the bacteria produces special attack enzymes — known as Cas9 — that carry around those stored bits of viral genetic code like a mugshot. When these Cas9 enzymes come across a virus, they see if the virus’s RNA matches what’s in the mugshot. If there’s a match, the Cas9 enzyme starts chopping the virus’s DNA up and neutralizing the threat. It looks a little like this:
/cdn.vox-cdn.com/uploads/chorus_asset/file/7725763/crispr_bg.jpg) (Shutterstock)
(Shutterstock)So that’s what CRISPR/Cas9 does. For a while, these discoveries weren’t of much interest to anyone except microbiologists — until a series of further breakthroughs occurred.
How did CRISPR revolutionize gene editing?
In 2011, Jennifer Doudna of the University of California Berkeley and Emmanuelle Charpentier of Umeå University in Sweden were puzzling over how the CRISPR/Cas9 system actually worked. How did the Cas9 enzyme match the RNA in the mugshots with that in the viruses? How did the enzymes know when to start chopping?
The scientists soon discovered they could “fool” the Cas9 protein by feeding it artificial RNA — a fake mugshot. When they did that, the enzyme would search for anything with that same code, not just viruses, and start chopping. In a landmark 2012 paper, Doudna, Charpentier and Martin Jinek showed they could use this CRISPR/Cas9 system to cut up any genome at any place they wanted.
While the technique had only been demonstrated on molecules in test tubes at that point, the implications were breathtaking.
Further advances followed. Feng Zhang, a scientist at the Broad Institute in Boston, co-authored a paper in Science in February 2013 showing that CRISPR/Cas9 could be used to edit the genomes of cultured mouse cells or human cells. In the same issue of Science, Harvard’s George Church and his team showed how a different CRISPR technique could be used to edit human cells.
Since then, researchers have found that CRISPR/Cas9 is ridiculously versatile. Not only can scientists use CRISPR to “silence” genes by snipping them out — they can also harness repair enzymes to substitute in desired genes into the “hole” left by the snippers (though this latter technique is trickier to pull off). So, for instance, scientists could tell the Cas9 enzyme to go snip out a gene that causes Huntington’s disease and insert a “good” gene to replace it.
/cdn.vox-cdn.com/uploads/chorus_asset/file/7724917/Artboard_1.jpg) (Javier Zarracina)
(Javier Zarracina)Gene editing itself isn’t new. Various techniques to knock out genes have been around for years. What makes CRISPR so revolutionary is that it’s incredibly precise: The Cas9 enzyme mostly goes wherever you tell it to go. And it’s incredibly cheap and easy: In the past, it might have cost thousands of dollars and weeks or months of fiddling to alter a gene. Now it might cost just $75 and only take a few hours. And this technique has worked on every organism it’s been tried on.
This has become one of the hottest fields around. In 2011, there were fewer than 100 published papers on CRISPR. In 2017, there were more than 14,000 and counting, with new refinements to CRISPR, new techniques for manipulating genes, improvements in precision, and more. “This has become such a fast-moving field that I even have trouble keeping up now,” says Doudna. “We’re getting to the point where the efficiencies of gene editing are at levels that are clearly going to be useful therapeutically as well as a vast number of other applications.”
There’s been intense legal battle over who, exactly, should get credit for this CRISPR technology — was Doudna’s 2012 paper the breakthrough, or was Zhang’s 2013 paper the key advance? Ultimately, the court ruled in February that the patent should go to Zhang and the Broad Institute, Harvard University and the Massachusetts Institute of Technology. In the July, the University of California and others on Doudna’s side said they were launching an appeal of the decision. But the important thing is that CRISPR has arrived.
So what can we use CRISPR for?
/cdn.vox-cdn.com/uploads/chorus_asset/file/7220933/shutterstock_81557950.jpg) (Shutterstock)
(Shutterstock)So many things. Paul Knoepfler, an associate professor at UC Davis School of Medicine, told Vox that CRISPR makes him feel like a “kid in a candy store.”
At the most basic level, CRISPR can make it much easier for researchers to figure out what different genes in different organisms actually do — by, for instance, knocking out individual genes and seeing what traits are affected. This is important: While we’ve had a complete “map” of the human genome since 2003, we don’t really know what function all those genes serve. CRISPR can help speed up genome screening, and genetics research could advance massively as a result.
Researchers have also discovered there are numerous CRISPRs. So CRISPR is actually a pretty broad term. “It’s like term fruit — it describes whole category,” said the Broad’s Zhang. When people talk about CRISPR, they are usually referring to the CRISPR/Cas9 system we’ve been talking about here. But in recent years, researchers like Zhang have found other types of CRISPR proteins that also work as gene editors. Cas13, for example, can edit DNA’s sister, RNA. “Cas9 and Cas13 are like apples and bananas,” Zhang added.
The real fun — and, potentially, the real risks — could come from using CRISPRs to edit various plants and animals. A recent paper in Nature Biotechnology by Rodolphe Barrangou and Doudna listed a flurry of potential applications in the future:
1) Edit crops to be more nutritious: Crop scientists are already looking to use CRISPR to edit the genes of various crops to make them tastier, or more nutritious, or better survivors of heat and stress. They could potentially use CRISPR to snip out the allergens in peanuts. Korean researchers are looking to see if CRISPR could help bananas survive a deadly fungal disease. Some scientists have shown that CRISPR can create hornless dairy cows — a huge advance for animal welfare.
Recently, major companies like Monsanto and Dupont have begun licensing CRISPR technology, hoping to develop valuable new crop varieties. While this technique won’t entirely replace traditional GMO techniques — which can transplant genes from one organism to another — CRISPR is a versatile new tool that can help identify genes associated with desired crop traits much more quickly. It could also allow scientists to insert desired traits into crops more precisely than traditional breeding, which is a much messier way of swapping in genes.
“With genome editing, we can absolutely do things we couldn’t do before,” says Pamela Ronald, a plant geneticist at University of California Davis. That said, she cautions that it’s only one of many tools for crop modification out there — and the challenge of successfully breeding new varieties could still take years of testing.
It’s also possible that these new tools could attract controversy. Foods that have had a few genes knocked out via CRISPR are currently regulated more lightly than traditional GMOs. Policymakers in Washington, DC, are currently debating whether it might make sense to rethink regulations here. This piece for Ensia by Maywa Montenegro delves into some of the debates CRISPR raises in agriculture.
2) New tools to stop genetic diseases: As the new Nature paper shows, scientists are now using CRISPR/Cas9 to edit the human genome and try to knock out genetic diseases like hypertrophic cardiomyopathy. They’e also looking at using it on mutations that cause Huntington’s disease or cystic fibrosis, and are talking about trying it on the BRCA-1 and 2 mutations linked to breast and ovarian cancers. Scientists have even shown that CRISPR can knock HIV infections out of T cells.
So far, however, scientists have only tested this on cells in the lab. There are still a few hurdles to overcome before anyone starts clinical trials on actual humans. For example: The Cas9 enzymes can occasionally “misfire” and edit DNA in unexpected places, which, in human cells, might lead to cancer or even create new diseases. As geneticist Allan Bradley, of England’s Wellcome Sanger Institute, told Stat, CRISPR’s ability to wreak havoc on DNA has been “seriously underestimated.”
And while there have also been major advances in improving CRISPR precision and reducing these off-target effects, scientists are urging caution on human testing. There’s also plenty of work yet to be done in actually delivering the editing molecules to particular cells — a major challenge going forward.
3) Powerful new antibiotics and antivirals: One of the most frightening public health facts around is that we are running low on effective antibiotics as bacteria evolve resistance to them. Currently, it’s difficult and costly to develop fresh antibiotics for deadly infections. But CRISPR/Cas9 systems could, in theory, be developed to eradicate certain bacteria more precisely than ever (though, again, figuring out delivery mechanisms will be a challenge). Other researchers are working on CRISPR systems that target viruses such as HIV and herpes.
4) Gene drives that could alter entire species: Scientists have also demonstrated that CRISPR could be used, in theory, to modify not just a single organism but to modify an entire species. It’s an unnerving concept called “gene drive.”
It works like this: Normally, whenever an organism like a fruit fly mates, there’s a 50-50 chance that it will pass on any given gene to its offspring. But using CRISPR, scientists can alter these odds so that there’s a nearly 100 percent chance that a particular gene gets passed on. Using this gene drive, scientists could ensure that an altered gene soon propagates throughout an entire population in short order:
/cdn.vox-cdn.com/uploads/chorus_asset/file/7724927/Artboard_3.jpg) (Javier Zarracina / Source: Oye et al 2014)
(Javier Zarracina / Source: Oye et al 2014)By harnessing this technique, scientists could (say) genetically modify mosquitoes to only produce male offspring — and then use gene drive to push that trait through an entire population. Over time, the population would go extinct. “Or you could just add a gene making them resistant to the malaria parasite, preventing its transmission to humans,” Vox’s Dylan Matthews explains in his story on CRISPR gene drives for malaria.
Suffice to say, there are also hurdles to overcome before this technology is rolled out en masse — and not necessarily the ones you’d expect. “The problem of malaria gene drives is rapidly becoming a problem of politics and governance more than it is a problem of biology,” Matthews writes. Regulators will need to figure out how to handle this technology, and ethicists will need to grapple knotty questions about its fairness.
5) Creating “designer babies.” This is the one that gets most of the attention. It’s not entirely far-fetched to think we might one day use CRISPR to edit the human genome — to eliminate disease, or select for athleticism, or for superior intelligence.
That said, scientists aren’t even close to being able to do this. We’re not even close to the point where scientists could safely make the complex changes needed to, say, improve intelligence in part because it involves so many genes. So don’t go dreaming of Gattaca just yet.
“I think the reality is we don’t understand enough yet about the human genome, how genes interact, which genes give rise to certain traits, in most cases, to enable editing for enhancement today,” Doudna said in 2015. Still, she added: “That’ll change over time.”
Wait, should we really create designer babies?
/cdn.vox-cdn.com/uploads/chorus_asset/file/7220099/shutterstock_299699342.jpg) (Shutterstock)
(Shutterstock)Given all the fraught issues associated with gene editing, many scientists are advocating a go-slow approach here. They are also trying to keep the conversation about this technology open and transparent, build public trust, and avoid some of the mistakes that were made with GMOs.
In February 2017, a report from the National Academy of Sciences said that clinical trials could be greenlit in the future “for serious conditions under stringent oversight.” But it also made clear that “genome editing for enhancement should not be allowed at this time.”
Society still needs to grapple with all the ethical considerations at play here. For example, if we edited a germline, future generations wouldn’t be able to opt out. Genetic changes might be difficult to undo. Even this stance has worried some researchers, like Francis Collins of the National Institute of Health, who has said the US government will not fund any genomic editing of human embryos.
In the meantime, researchers in the US who can drum up their own funding, along with others in UK, Sweden, and China are moving forward with their own experiments.
Further reading
- At Vox, we reported on 2 new CRISPR tools overcome the scariest parts of gene editing.
- Ezra Klein interviewed UC-Berkeley’s Jennifer Doudna, one of the leading CRISPR researchers, on his podcast in October.
- Michael Specter’s “Rewriting the Code of Life” in the New Yorker.
- Carl Zimmer has been on the CRISPR beat for a long time. His 2015 piece in Quanta is well worth reading.
- Sharon Begley has also been on the CRISPR beat, and recently unpacking the latest studies on potential harms of gene editing.
- In 2016, Nature explored some of the subtle limitations of CRISPR — and the search for additional gene-editing tools. And this earlier Nature piece by Heidi Ledford is a delightfully wonky dive into the ways researchers could use CRISPR to explore the genome. It’s also worth checking out this paper listing all the different future applications of CRISPR.
Author: Brad Plumer
Read More